bsl 2 checklist
O Are Biomedical Waste containers not overfilled. This form is intended to aid in assessing a laboratory as appropriate to do BSL-2 studies in locations other than a registered research institution eg.
Dispose of used gloves with other.
. Samples are stored in a secure location. You can review your biosafety inspection results and respond to any findings within the online EHSA system for inspections taking place in. Before leaving the laboratory.
To ensure laboratories meet basic requirements at the federal state and local levels for BSL-2 practices and containment the BSL-2 checklist must be completed for each space designated at BSL-2. Samples are properly labeled. The universal biohazard symbol the rooms Animal Biosafety Level the supervisors or other responsible personnels name and telephone number PPE require ments general occupational health requirements e.
In addition BSL-2 laboratory workers should. Queries are based on the Biosafety Level 2 Section of the Biosafety in Microbiological and Biomedical Laboratories 4th Edition May 1999 Circle the response that best describes the laboratory in. This is the Biosafety Level 2 BSL-2 laboratory inspection checklist.
O Are Biohazard Waste containers containing infectious materials covered. Leading Manufacturer of Cleanroom Supplies Contamination Control Products. Laboratory Assistance Visit Checklist BSL2 laboratories.
Risk Group 2 Material Storage 1. Experiment areas of lesser biohazard potential are carefully demarcated. Risk Group 2 material is segregated from other materials.
Biosafety Level OneTwo BSL-12 Inspection Checklist ASUs research and teaching laboratories that work with biohazards are required to have annual inspections by EHS Biosafety and Biosecurity professionals. Lab Biosafety Level 2 Checklist. A procedure-based approach comprising the practices personal protective equipment facilities and implementation of your laboratorys Standard Operating Procedures SOPs must be taken when evaluating safety and compliance.
BSL-2 Commissioning Check-list Revision date. Recombinant DNA activity requiring BSL-2 physical containment including animal studies that involve the construction of transgenic animals. April 16 1998 Date.
Remove gloves and wash hands when work with hazardous materials has been completed and before leaving the laboratory. This biosafety level covers laboratories that work with agents associated with human diseases ie. Each laboratory space where biohazardous materials are used is assigned one of 3 internationally recognized biosafety levels or BSL.
Biosafety Level 2 Section of the Biological Safety in Microbiological Biomedical and Laboratories 5thedition. A procedure-based approach comprising the practices personal protective equipment facilities and implementation of your laboratorys Standard Operating Procedures SOPs must be taken when evaluating safety and compliance. The following checklist is based on the Biosafety Level 2 section of Laboratory Biosafety Manual 3rd.
Not found sitting directly on the floor. A Self- Assessment Safety Checklist. Laboratory Biosafety Compliance Inspection Checklist.
These questions are based on the Biosafety Level 2 section of Biosafety in Microbiological and Biomedical Laboratories. This checklist will be used during the. Doors are signed with an international biohazard symbol.
Instructions Using the checklist below inspect your lab and note any deficiencies that need to be addressed the PI may assign a senior lab member to complete the checklist but the PI must reviews date and sign the checklist. Laboratory Biosafety Compliance Inspection Checklist Biosafety Level 2. Sign and date the completed checklist.
Revised May 2021 Yes No NA Response. Ad Over 20 Years As A Leading Manufacturer Of Cleanroom Supplies Wiping Solutions. Examples of agents typically worked with in a BSL-2 include equine encephalitis viruses and HIV as well as Staphylococcus aureus staph infections.
Import Permit Program ABSL-2 Inspection Checklist Page 2 of 11. C4-c In addition BSL-2 laboratory workers should. Taking into account the nature of the processing assist the controller by appropriate.
Biosafety Level 2 Laboratory Audit 3 Satisfactory Needs Improvement NA Human Materials 1. Biosafety Level 2 builds upon BSL-1. Laboratory Biosafety Compliance Inspection Checklist.
Pathogenic or infections organisms that pose a moderate health hazard. High school laboratory medical office diagnostic lab. In 2007 the CDC enhanced requirements for all work at BSL-2.
Standard Operating Procedures SOPs. O Is biomedical waste awaiting autoclaving placed within a leak-proof secondary container ie. This checklist is recommended for use as a tool for self-assessment and is.
April 2021 Revision 2 421 BSL-2 Commissioning Checklist Page 2 of 2 contaminated materials if they are removed from the laboratory for autoclaving. Respect the conditions for engaging another processor referred to in paragraphs 2 and 4 of Article 28 processor of the EU General Data Protection Regulation 2016679. Consumption must not be permitted in laboratory areas.
C4-c In addition BSL-2 laboratory workers should. Non-recombinant cell andor tissue culture systems that require this level of containment. Biosafety Levels BSL Research and teaching activities involving infectious agents requires prior approval by the Institutional Biosafety Committee IBC via the Biohazard Use Authorization BUA review process.
BioSafety Level 2 Checklist. You can use this checklist to help prepare for your biosafety lab inspection and to easily see the requirements for BSL-2 laboratories. Laboratory Assistance Visit Checklist BSL2 laboratories.
Biosafety Level 2 Checklist BSL-2 Loading. Persons must wash their handsafter working with potentially hazardous materials and. 07212017 - 2100.
Not wash or reuse disposable gloves. O Are Biomedical Waste boxes found to be lined with the correct bag. Microorganisms of low biohazard potential such as those in Risk Group 2 or BSL-2.
BSL-2 is suitable for work involving agents that pose moderate hazards to personnel and the. Eating drinking smoking handling contact lenses applying cosmetics and storing food for human. Mechanical pipetting devices are available and used for all pipetting.
This checklist is recommended for use as a tool for self-assessment and is.

Pdf Bsl2 Audit And Certification Program An Effort To Harmonize And To Raise Standards In Both Laboratory Infrastructure And Biosafety Practices In Singapore Semantic Scholar
Biosafety Level 2 Laboratory Self Audit Checklist

Biosafety Level 2 Inspection Checklist University Of Southern

Biosafety Level 2 Inspection Checklist University Of Southern
Biohazard Self Audit Checklist

Biosafety Level 2 Inspection Checklist University Of Southern
Laboratory Information Biosafety Level 2 Checklist Bei Resources

Bsl 2 Checklist Reference Aimst Pdf Laboratories Personal Protective Equipment
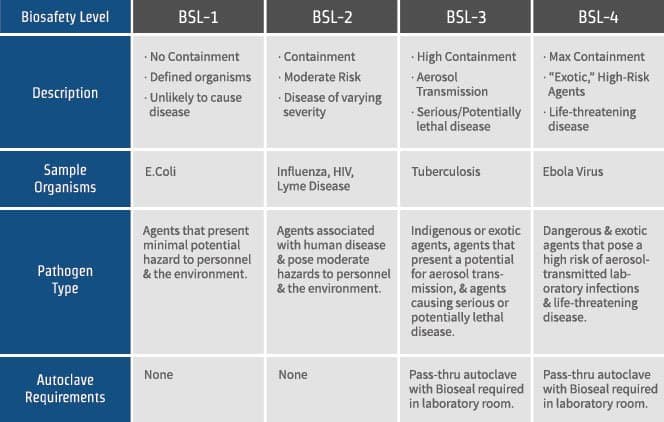
Biosafety Levels 1 2 3 4 What S The Difference

Basic Biosafety Inspection Checklist

Biosafety Level 2 Inspection Checklist University Of Southern
Comments
Post a Comment